About Water
Water (Chemical Formula is H O) is a transparent fluid which forms the 2 world's streams, lakes, oceans, and rain, and is the major constituent of fluid of organisms. As a chemical compound, a water molecule contains one oxygen and two hydrogen atoms that are connected by covalent bonds, water is a liquid at standard ambient temperature and pressure.
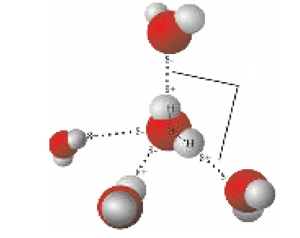
Water is sometimes referred to as the “universal solvent”. This term is used as water has unique characteristics that allow it to break the bonds of larger more complex compounds. For example, place a small teaspoon of sugar or salt in a glass of water, stir vigorously and it will dissolve easily. Although the water in your home is clear, it is not pure. Being a wonderful solvent, water is very receptive and dissolves small amounts of the soluble minerals it comes into contact with in nature. Water contains elements essential for healthy living, including calcium and magnesium.
To understand how our technology works let’s first remember some basic facts about water. Water is sometimes referred to as the “universal solvent”. This term is used as water has unique characteristics that allow it to break the bonds of larger more complex compounds. For example, place a small teaspoon of sugar or salt in a glass of water, stir vigorously and it will dissolve easily.
Although the water in your home is clear, it is not pure. Being a wonderful solvent, water is very receptive and dissolves small amounts of the soluble minerals it comes into contact with in nature. Water contains elements essential for healthy living, including calcium and magnesium.
What is Hard Water…?
Hard water is water that has high mineral content. Hard water is formed when water percolates through deposits of calcium and magnesium-containing minerals such as limestone, chalk and dolomite. Hard water generally poses serious problems in industrial settings, where water hardness is monitored to avoid costly breakdowns in boilers, cooling towers, and other equipment that handles water. In domestic settings, hard water is often indicated by a lack of suds formation when soap is agitated in water, and by the formation of lime scale in kettles and water heaters. Water's hardness is determined by the concentration of multivalent cations in the water. Multivalent cations are positively charged metal complexes with a charge greater than 1+. 2+ Usually, the cations have the charge of 2+. Common cations found in hard water include Ca 2+ and Mg . These ions enter a water supply by leaching from minerals within an aquifer. Common calcium-containing mineral is calcite.
Hardness in Water
Hardness in water is caused by Calcium and Magnesium. Conventional water softeners remove the essential Calcium and Magnesium macrominerals from the water supply, by replacing them with Sodium. One of the benefits of the solutions provided by Fluid Dynamics is that they do not remove these essential macrominerals.
Magnesium
While contributing to water hardness, Magnesium does not deposit as scale.
Catalytic Technology
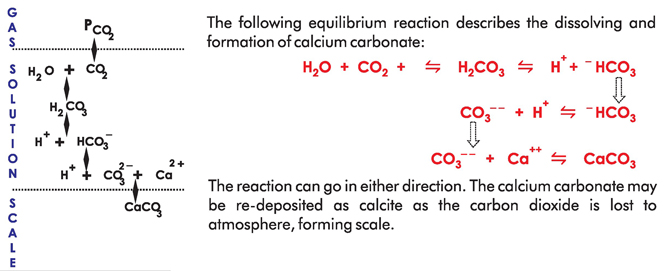
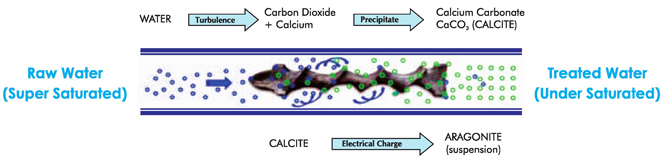
When water enters in the device with recommended flow it creates turbulence and due to which ++ Ca and CO (Carbon Dioxide) are forced to generate CaCO (Calcium Carbonate). Due to this 2 3 ++ process, now in the less presence of free Ca ions the nature of water becomes soft. Remember in water CaCO is also already available before turbulence. It is in calcite form which by nature is 3 adhesive and the main reason of scaling. When this CaCO after turbulence passes through the 3 Catalytic device of Fluid Dynamics it strikes (hits forcibly) to the Special Alloy present in the device and gets an small electric charge which converts it's calcite crystals in to aragonite crystals. These are suspended and non-adhesive particles of CaCO and are called aragonite particles. This 3 aragonite form of CaCO does not form scaling and ultimately the water becomes soft and scale 3 free. When CaCO calcite form crystals convert in to aragonite form crystals then the water comes 3 in the state of under saturation form the super saturation. When water is treated through Fluid Dynamics Catalytic Technology device and it tries to come in saturation form from under saturation form it involves the old existing scaling in the pipe lines/supply system and mixed with in. Due to this kind of nature this technology is claimed to prevent Hard Water Scaling as well as cure of old existing scaling in the system/pipe line.

Conclusion:
- 1. Solution of Hard Water : Water behaves as soft water because the main factor responsible for ++ hard water ca is mostly converted into CaCO in the process of catalytic water treatment. 3
- 2. Solution of scaling formation : The treated water is 100% scale-free because all the CaCO 3 (Calcite form - Hard Calcium Crystal) are converted completely into it's Aragonite form (suspended form - Soft Calcium Crystal).
- 3. Solution of Existing Scaling : The treated water removes all the existing scaling because of the under-saturated condition.
- 4. Treated water can be stored for several months.
Calcium Carbonate
As we all know H2O can exist in various forms; Ice, Water and Steam.
Just as water can exist in various forms, so can Calcium Carbonate. The three forms of Calcium Carbonate are:
Calcite
Under certain circumstances ( temperature rise or an increase in pH levels), water is forced to discharge Calcium Carbonate. The Calcium Carbonate discharged in this way is Calcite, a hard scale. Calcite will accumulate on the nearest receptive surface, typically metallic. In Pictures shown to the untreated form of calcium carbonate. This is the hard scale deposited by untreated water.



Under certain circumstances ( temperature rise or an increase in pH levels), water is forced to discharge Calcium Carbonate. The Calcium Carbonate discharged in this way from untreated water is Calcite, a hard scale. The discharge will accumulate on the nearest receptive surface, typically metallic.
Aragonite
Aragonite is a form of Calcium Carbonate crystal that, unlike Calcite, prefers to stick to itself and grow attracting more Calcium. It remains suspended in water rather than depositing onto metal surfaces.



In Picture is shown the treated form of calcium carbonate. Unlike calcite, aragonite stays in suspension and is carried through the system to the drain. As a result the system does not scale.
Aragonite is a form of Calcium Carbonate crystal that, unlike Calcite, prefers to stick to itself and grow attracting more Calcium. It remains suspended in water rather than depositing on surfaces.
Vaterite
Vaterite is not found in natural water.
Events that cause scaling
Hot Water Scaling
In untreated hard water systems when contact is made with the hot surfaces found in water heaters, humidifiers, kettles, boilers, heat exchangers etc. scale will be deposited.
Calcium is inversely soluble, meaning that as
the water temperature rises, the
amount of Calcium that can be held in solution
decreases. When this occurs in hard water
systems the excess Calcium that’s been displaced
by the temperature rise bonds with available
bicarbonate to form Calcium Carbonate (in the
Calcite form). This newly formed Calcium
Carbonate then deposits as scale on the nearest
receptive surface.
Cold Water Scaling
In untreated hard water systems a rise in pH will also cause scaling. Carbon Dioxide dissolved in water under pressure takes the form of Carbonic acid. When the water pressure drops, at a faucet for example, the Carbonic acid gasses off in the form of Carbon Dioxide causing an instant pH rise ( making the water less acidic). Examples of pressure drops in systems that can produce pH related cold water scaling include nozzles, faucets and shower heads.
The Fluid Dynamics Solution
Now that we’ve covered Calcium Carbonate, the two forms it can take in natural water and the events that trigger scale deposits, we are able to explain the mechanism behind our successful technology.
As water passes through our product it is subjected to a turbulent interaction with a catalytic core made of a proprietary alloy. The dissimilar metals and the water create a battery effect generating a very small electrical current.
This electric current causes an important change to take place. A percentage of the Calcium and Bicarbonate in the water comes out of solution and goes into suspension forming Calcium Carbonate in the Aragonite state.
The microscopic Aragonite crystal formations remain suspended in the water and pass harmlessly through the system. Changes in temperature or pH no longer lead to hard scale deposits. The Calcium Carbonate that is placed in suspension as stable Aragonite crystals is no longer available in solution to be deposited as Calcite.
As the process repeats itself, newly created Aragonite crystal formations are continually produced thus preventing scale deposits. The process also continues to gradually remove any scale that may have accumulated in the past. This is due to the water now being under saturated with dissolved calcium and bicarbonate increasing its capacity to act as a solvent.
For a more in depth explanation that covers saturation ratios and looks at the chemistry behind the whole process please click this “In Depth Science” link.



